Cell Motility
In multicellular tissues, such as those found in animals and humans, individual cells employ a variety of locomotion mechanisms to maneuver through spaces in the extracellular matrix and over the surfaces of other cells. Examples are the rapid movement of cells in developing embryos, organ-to-organ spreading of malignant cancer cells, and the migration of neural axons to synaptic targets. Unlike single-celled swimming organisms, crawling cells in culture do not possess cilia or flagella, but tend to move by coordinated projection of the cytoplasm in repeating cycles of extension and retraction that deform the entire cell. The digital videos presented in this gallery investigate animal cell motility patterns in a wide variety of morphologically different specimens.
Albino Swiss Mouse Embryo Fibroblasts (3T3 Line)
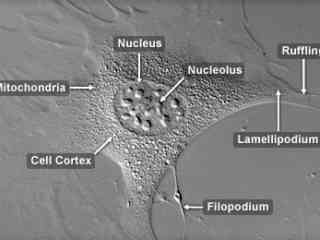
Explore how the cells in this active fibroblast interact with each other in culture. The 3T3 cell line is an important fibroblast culture, widely utilized in laboratory research, which was established from disaggregated tissue of an albino Swiss mouse (Mus musculus) embryo. In culture, 3T3 cells double in approximately 24 hours to eventually form confluent monolayers that display contact inhibited cell motility.
Bovine Pulmonary Artery Endothelial Cells (BPAE Line)
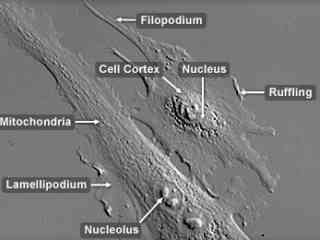
The BPAE cell line was initiated in 1979 from the main stem of a pulmonary artery belonging to a young cow (Bos taurus). The endothelial cells are positive for bovine diarrhea virus and for angiotensin converting enzyme (ACE), an enzyme involved in the maintenance of blood pressure and volume. BPAE cells are often utilized in hypertension research, as well as studies of atherosclerosis and coronary heart disease.
Embryonic Rat Thoracic Aorta Medial Layer Myoblasts (A-10 Line)
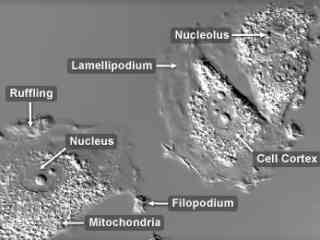
Derived from the thoracic aorta of an embryonic rat, A-10 cells have been important in a variety of investigations as cell motility models for in vitro wounds. During migration, the cells display a significant amount of ruffling at the outer membrane edges along with numerous filopodia projecting into the culture medium. The cells also exhibit highly prominent cytoskeletal stress fibers that often traverse the entire cytoplasm.
Human Bone Osteosarcoma Epithelial Cells (U2OS Line)
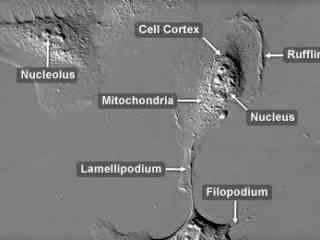
Originally known as the 2T line, the U2OS cell line was cultivated from the bone tissue of a fifteen-year-old human female suffering from osteosarcoma. Established in 1964, the original cells were taken from a moderately differentiated sarcoma of the tibia. U2OS cells exhibit epithelial morphology and are positive for insulin-like growth factor I (IGF-I) and insulin-like growth factor II (IGF II) receptors.
Madin-Darby Bovine Kidney Epithelial Cells (MDBK Line)
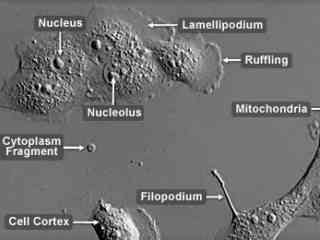
The Madin-Darby bovine kidney (MDBK) line was derived from the renal tissue of an adult steer (Bos Taurus) in 1957. The epithelial line is utilized in laboratories around the world for a variety of applications, but is perhaps most significant for its usage to grow attenuated viruses for vaccine production. The cells are susceptibled to bovine diarrhea virus, vesicular stomatitis (Indiana strain), infectious bovine rhinotracheitis virus, bovine parvovirus, bovine adenovirus I and III, and parainfluenza virus 3
Madin-Darby Ovine Kidney Epithelial Cells (MDOK Line)
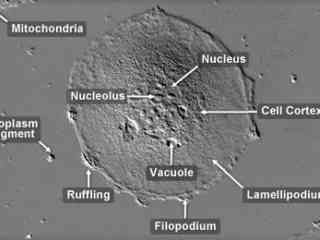
The Madin-Darby ovine kidney (MDOK) cell line was established from the renal tissue of a male sheep (Ovis aries). The line exhibits typical epithelial morphology and is susceptible to several viruses including vesicular stomatitis (Indiana and New Jersey strains), infectious bovine rhinotracheitis, and sheep bluetongue virus. MDOK cells exhibit significant contact inhibition of migration and tend to gather together into small colonies in culture.
Male Human Lung Carcinoma Epithelial Cells (A-549 Line)
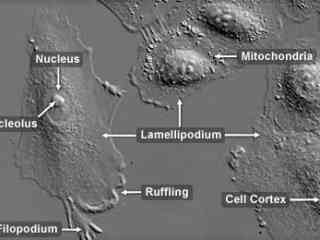
The A-549 cell line was originally cultivated in 1972 from the human lung carcinoma of a 58-year-old Caucasian male. The line is commonly used to investigate a wide range of respiratory ailments, such as viral infections capable of inducing asthma, tissue damage linked to asbestos exposure, and smoking-related emphysema. Adherent and epithelial in morphology, A-549 cells have also been widely employed as a model system to study molecular mechanisms that operate during malignant cell migration.
Normal African Green Monkey Kidney Fibroblast Cells (CV-1 Line)
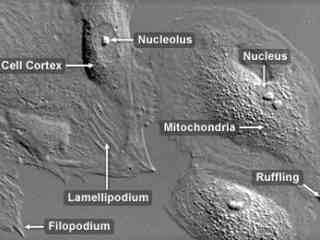
The CV-1 cell line was initiated in 1964 with a tissue section excised from the kidney of an adult male African green monkey (Cercopithecus aethiops). The fibroblast line was originally utilized in research focusing on the transformation of the cancer-causing Rous sarcoma virus (RSV), but now is popular as a host for acquired immunodeficiency disease (AIDS) research, as well as transfection experiments with simian virus 40 (SV40) and recombinant plasmid vectors.
Normal Rabbit Kidney Epithelial Cells (RK13 Line)
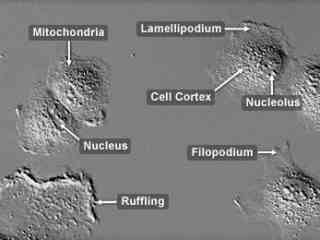
Initiated from the kidney of a 5-week-old rabbit (Oryctolagus cuniculus), RK13 cells exhibit epithelial characteristics and are positive for keratin by immunoperoxidase staining. The established cell line is commonly used to isolate viruses and as transfection hosts. The cells have proven susceptible to infection with the B virus, herpes simplex, pseudorabies virus, vaccinia, rabbitpox, myxoma, simian adenoviruses, and rubellavirus
Opossum Kidney Cortex Proximal Tubule Epithelial Cells (OK Line)
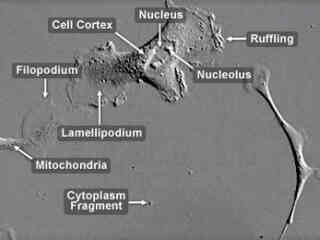
The OK cell line was initiated from the kidney of an adult female North American opossum (Didelphis marsupialis virginiana) and was originally intended for use as a source of X chromosomes for studies of X inactivation. The line was soon discovered, however, to display many characteristics of kidney proximal tubular epithelial cells and has since been commonly utilized as a cell culture model for the cell type.
Raccoon Uterus Fibroblast Cells (PL 1 Ut Line)
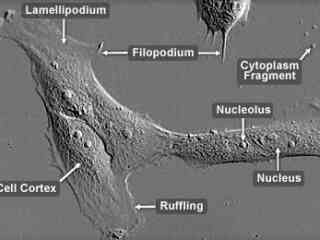
PL 1 Ut cells exhibit fibroblast-like morphological characteristics and are susceptible to an array of viruses, including herpes simplex virus, reovirus 3, and vesicular stomatitis (Ogden strain). The cells are commonly utilized in the propagation of such viruses for research purposes, and have been particularly useful in investigations of feline and canine viral diseases. The PL 1 Ut cell line was cultivated from the uterine tissue of an adult female North American raccoon (Procyon lotor).
Rhesus Monkey Kidney Epithelial Cells (LLC-MK2 Line)
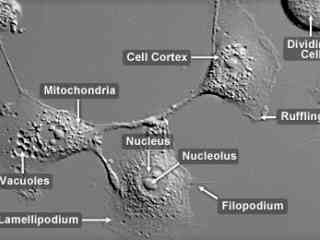
In the mid-1950s, the LLC-MK2 cell line was established from a pooled cell suspension prepared from the kidney tissue of six adult rhesus monkeys (Macaca mulatto). The cells exhibit epithelial morphology and produce the protease plasminogen activator that typically initiates the process of fibrinolysis by converting plasminogen to plasmin. LLC-MK2 cells have been utilized in the production of mumps vaccines and in the isolation of parainfluenza viruses, and are communly used as transfection hosts.
Albino Swiss Mouse Embryo Moloney Murine Leukemia Virus Transfected Cells (CRE BAG 2 Line)
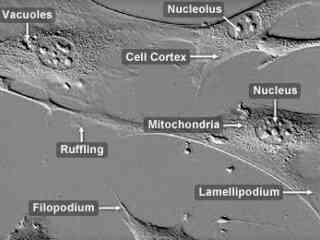
The CRE BAG 2 cell line was established from the NIH 3T3 embryonic Swiss mouse fibroblast cell line, which was transfected with Moloney murine leukemia virus-derived proviral genomes carrying complementary mutations in the gag-pol or env regions. CRE BAG2 cells produce a beta-galactosidase-transducing vector (BAG) and are similar to the psi 2 BAG alpha line. The CRE BAG 2 line can be utilized to package vectors derived from murine leukemia viruses and is positive for reverse transcriptase.
African Water Mongoose Skin Fibroblast Cells (A. P. Mongoose Line)
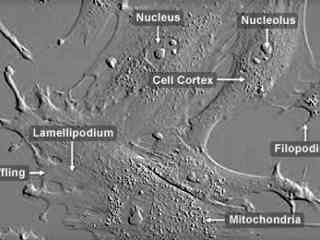
The A. P. Mongoose cell line was established at The Naval Biosciences Laboratory (NBL) in Oakland, California from the skin of an African water mongoose (Atilax paludinosus). The cells exhibit fibroblast morphology, and similar to other fibroblast lines, are among the easiest cells to grow in culture. Cell biologists hypothesize that the ability of fibroblasts to grow so readily outside of the body is associated with their central role in the healing of wounds, which necessitates their proliferation when confronted with injury or other less than optimal conditions.













