Phase Contrast Microscopy
Differences in light absorption are often negligible between living cells and their surrounding nutrient medium, as well as between the various intracellular components and plasma membranes, rendering these entities barely visible when observed by brightfield illumination. Phase contrast microscopy takes advantage of minute refractive index differences within cellular components and between unstained cells and their surrounding aqueous medium to produce contrast in these and similar transparent specimens.
Review Articles
Introduction to Phase Contrast Microscopy (日本語)
A mechanism to translate variations in phase into corresponding changes in amplitude.
Phase Contrast Microscope Configuration
Phase contrast optical components can be added to virtually any brightfield microscope.
Specimen Contrast in Optical Microscopy
Using phase-related optical techniques to increase specimen contrast.
Apodized Phase Contrast
Advanced phase ring technology that enables a reduction in image halo artifacts.
Interactive Tutorials
-
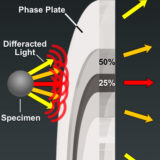
Apodized Phase Contrast
Explore how specimen size affects the angle of diffracted light rays that pass through apodized phase plates.
-
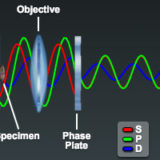
Optical Pathways in the Phase Contrast Microscope
Examine the light pathways through a phase contrast microscope and learn how these systems dissect the incident electromagnetic wave into a surround (S), diffracted (D), and resultant particle (P) component.
-

Phase Contrast Microscope Alignment
Learn how to align a phase contrast microscope and examine variations in specimen appearance through the eyepieces (at different magnifications) when the condenser annulus is shifted into and out of alignment with the phase plate in the objective.
-
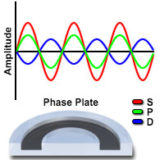
Positive and Negative Phase Contrast
This interactive tutorial explores relationships between the surround (S), diffracted (D), and resulting particle (P) waves in brightfield as well as positive and negative phase contrast microscopy.
-

Shade-Off and Halo Phase Contrast Artifacts
Explore shade-off and halo artifacts, where the observed intensity does not directly correspond to the optical path difference (refractive index and thickness values) between the specimen and the surrounding medium.
-
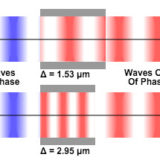
Specimen Optical Path Length Variations
Explore the effects of changes to refractive index and thickness on optical path length, and discover how two specimens can have different combinations of these variables but still display the same path length.
Related Nikon Products
Optics
World-class Nikon objectives, including renowned CFI60 infinity optics, deliver brilliant images of breathtaking sharpness and clarity, from ultra-low to the highest magnifications.
Objective Selector
Filter, find, and compare microscope objective lenses with Nikon's Objective Selector tool.
Inverted Microscopes
Serving as either as a standalone system or by powering the core of complex, multimodal imaging systems, Nikon’s inverted microscopes ensure the highest imaging results for every experiment.
Upright Microscopes
Legendary Nikon optics in each Nikon upright microscope guarantee outstanding imaging results for clinical applications to multiphoton imaging.
Selected Literature References
Differential Interference Contrast (DIC)
Contrast enhancement using interference of polarized light wavefronts.
General Phase Contrast Literature Sources
A listing of pertinent books, review articles, and research reports on phase contrast.
Phase Contrast Microscopy
Using refractive index variations to produce optical contrast in transparent specimens.
Specimen Contrast in Microscopy
Examine the origins of contrast in a wide spectrum of specimens.













