Live-Cell Imaging
An increasing number of investigations are using live-cell imaging techniques to provide critical insight into the fundamental nature of cellular and tissue function, especially due to the rapid advances that are currently being witnessed in fluorescent protein and synthetic fluorophore technology.
Review Articles
Maintaining Live Cells on the Microscope Stage
A discussion of pH, atmosphere, temperature, media, osmolarity, buffers, and antibiotics.
Live-Cell Imaging Culture Chambers
Chambers must offer excellent optical properties while maintaining healthy cells.
Introduction to Fluorescent Proteins
Genetically-encoded fluorescent probes that are revolutionizing live-cell imaging.
Correcting Focus Drift in Live-Cell Microscopy
Review software and hardware solutions for combating focus drift in time-lapse imaging.
Optical System and Detector Requirements for Live-Cell Imaging
Considerations are detector sensitivity, speed of image acquisition, and photobleaching.
The Automatic Microscope
Discussion includes shutters, filter wheels, autofocus, light sources, and stage control.
Imaging Fluorescent Proteins
Filter requirements, photobleaching, objective choice, highlighters, and multicolor imaging.
Nikon Perfect Focus System (PFS)
Explore Nikon's high-speed solution for maintaining stable focus in live-cell imaging.
Interactive Tutorials
-
Adjustment of Objective Correction Collars
Learn how to adjust an objective correction collar to minimize spherical aberration.
-
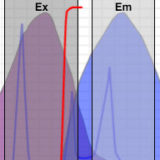
Choosing Filter Combinations for Fluorescent Proteins
Identification of critical filter parameters for imaging fluorescent proteins.
-
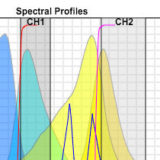
Choosing Fluorescent Proteins for Dual Labeling Experiments
Interactive tutorial used to optimize pairing of two fluorescent proteins.
-
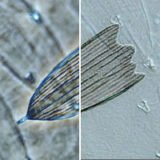
Comparison of Phase Contrast & DIC Microscopy
Examples of the same specimen viewed in either phase contrast or DIC.
-

DIC Microscope Component Alignment
Examine conoscopic and orthoscopic viewfields in DIC microscopy.
-

Focus and Alignment of Mercury and Xenon Arc Lamps
Explore alignment and focusing of the arc lamp in a mercury or xenon burner, which simulates how the lamp is adjusted in a real microscope.
-
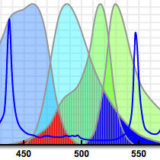
Förster (Fluorescence) Resonance Energy Transfer with Fluorescent Proteins
Use this tool to determine the optimum fluorescent protein pairs for FRET.
-
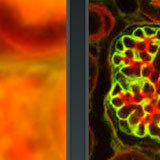
Laser Scanning Confocal Microscopy
A virtual microscope tutorial featuring a wide variety of specimens.
-
Matching Camera to Microscope Resolution
Vary numerical aperture, magnification, and video coupler size to match camera resolution.
-
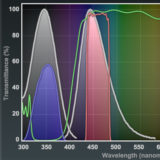
Matching Fluorescent Probes with Nikon Fluorescence Filter Blocks
Explore the various fluorophores that can be imaged with Nikon filter sets.
-
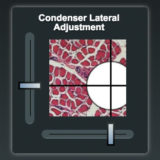
Microscope Alignment for Köhler Illumination
Learn how to adjust a microscope to examine specimens in Köhler illumination.
-
Optical Sectioning with de Sénarmont DIC Microscopy
At high numerical apertures, DIC can be used for optical sectioning.
-
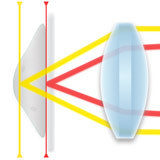
Perfect Focus Offset System Mechanics
Learn how the offset lens system enables the operation of the Nikon PFS.
-

Phase Contrast Microscope Alignment
Learn how to align a phase contrast microscope and examine variations in specimen appearance through the eyepieces (at different magnifications) when the condenser annulus is shifted into and out of alignment with the phase plate in the objective.
-
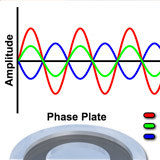
Phase Plate Configuration Effects on Specimen Contrast
Examine contrast variations induced by altering phase plate absorption properties.
-
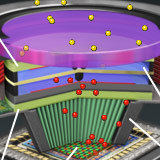
Proximity-Focused Image Intensifiers
Light amplification with a micro-channel plate and photocathode.
-
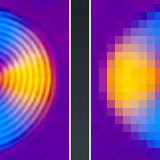
Spatial Resolution in Digital Imaging
Spatial resolution refers to the number of pixels utilized in construction of the image.
-
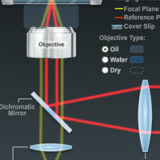
The Nikon Perfect Focus System (PFS)
Examine how the Nikon PFS system operates to maintain stable focus in live-cell imaging.
Galleries
Confocal Microscopy
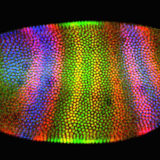
Enjoy the beauty of autofluorescence in thick sections of animal and plant tissues.
Movies
Differential Interference Contrast (DIC)
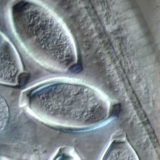
Compare specimen contrast using these complementary imaging techniques.
Image Comparisons • Movies
Fluorescence Microscopy
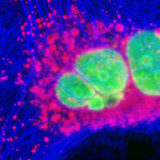
Cells and tissues examined with synthetic fluorophores in fluorescence microscopy.
Images
Phase Contrast
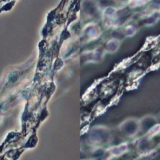
Specimen viewfields examined using positive and negative phase contrast.
Image Comparisons
Selected Literature References
Live-Cell Imaging
Basic concepts in maintaining and imaging living cells on the microscope stage.
Focus Drift in Optical Microscopy
Focus drift describes the inability to maintain focus over an extended period of time.
Fluorescent Proteins
Fluorescent proteins are currently the probes of choice for live-cell imaging.
Fluorescence Recovery after Photobleaching
FRAP is used to determine the mobility of fluorescently tagged proteins in live cells.
Chromophore-Assisted Light Inactivation
CALI is a useful technique for selectively deactivating target molecules.
Live-Cell Imaging Chambers
Chambers specially designed to keep cells alive and healthy on the microscope stage.
Electron-Multiplying CCD Cameras
EMCCDs are the detector system of choice for live-cell imaging in real-time applications.
Fluorescent Protein Biosensors
Genetically-encoded FRET biosensors are useful in detecting a many biological events.
Synthetic Fluorophores and Hybrid Tags
A number of fluorophores have been developed for imaging living and fixed cells.
FRET Microscopy with Spectral Imaging
Excellent for ratiometric analysis of photobleaching and spectral imaging FRET.