Fluorescence Microscopy
In the rapidly expanding fields of cellular and molecular biology, widefield and confocal fluorescence illumination and observation is becoming one of the techniques of choice. These techniques, which are almost universally employed in both the medical and biological sciences, have spurred the development of more sophisticated microscopes and numerous fluorescence accessories.
Review Articles
Introduction to Fluorescence Microscopy (日本語)
Basic equipment and techniques necessary for observing specimens in fluorescence.
Basics of FRET Microscopy
Using fluorescence to examine dynamic interactions between probes in living cells.
Fluorescence in situ Hybridization
Referred to as FISH, the technique is used primarily for chromosomal analysis.
Introduction to Fluorescent Proteins (日本語)
Genetically-encoded fluorescent probes that are revolutionizing live-cell imaging.
Stereomicroscopy Fluorescence Illumination
Fluorescence Illuminators enable examination of large specimens in stereomicroscopy.
Optical System and Detector Requirements for Live-Cell Imaging
Considerations are detector sensitivity, speed of image acquisition, and photobleaching.
Total Internal Reflection Fluorescence (TIRF) Microscopy (日本語)
TIRF restricts the excitation and detection of fluorophores to a thin region of the specimen.
Multiphoton Microscopy
Mode-locked pulsed lasers are used for deep tissue imaging and optical sectioning.
Fluorescence Filter Combinations
Discussion of the properties of various fluorescence filter combinations.
Imaging Fluorescent Proteins
Filter requirements, photobleaching, objective choice, highlighters, and multicolor imaging.
Laser Safety
Increased use of lasers demands attention to safety features in optical microscopy.
Interactive Tutorials
-
Matching Fluorescent Probes with Nikon Fluorescence Filter Blocks
Explore the various fluorophores that can be imaged with Nikon filter sets.
-

Stereomicroscopy Fluorescence
Explore the light paths in Nikon's SMZ1500 stereomicroscope equipped for fluorescence illumination using an intermediate tube and external lamphouse.
-
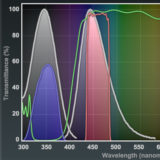
Blue Excitation
The Nikon blue excitation fluorescence filter combinations include bandpass and longpass sets having both broad and narrow excitation bandwidths.
-
Blue-Violet Excitation
Exploring how the variations in the excitation and emission filter spectral profiles, as well as those of the dichromatic mirrors, affect signal levels, overall filter performance, and image contrast in combinations designed for excitation of fluorophores in the blue-violet region.
-
Dual Band Excitation
Explore how the variations in the excitation and emission filter spectral profiles affect signal levels, overall filter performance, and image contrast in combinations designed for dual excitation of fluorophores in the ultraviolet and blue or blue and green regions.
-
Violet Excitation
Discussion of the properties of various fluorescence filter combinations.
-

Focus and Alignment of Mercury and Xenon Arc Lamps
Explore alignment and focusing of the arc lamp in a mercury or xenon burner, which simulates how the lamp is adjusted in a real microscope.
-
Green Excitation
Explore how the variations in the excitation and emission filter spectral profiles affect signal levels, overall filter performance, and image contrast in combinations designed for excitation of fluorophores in the green (510-560 nanometers) spectral region.
-
Laser Scanning Confocal Microscopy
A virtual microscope tutorial featuring a wide variety of specimens.
-
Triple Band Excitation
Filter sets for DAPI, FITC, and TRITC or Texas Red.
-
Ultraviolet Excitation
Examine specimen contrast with longpass and shortpass filter sets.
-
Yellow Excitation
Specimen contrast variations with narrow and wide bandpass filter combinations.
Selected Literature References
Autofluorescence
Natural fluorescence in plants and animals that can also be introduced by fixatives.
Confocal Microscopy
Imaging with laser excitation and pinhole detection of fluorescence.
Deconvolution Microscopy
A powerful tool for three-dimensional analysis of complex biological specimens.
Fluorescence Microscopy
Examining specimens labeled with molecules that absorb light and emit fluorescence.
Fluorescence Recovery after Photobleaching
FRAP is used to determine the mobility of fluorescently tagged proteins in live cells.
Fluorescence Resonance Energy Transfer
Probing molecular interactions in living cells using fluorescent proteins and fluorophores.
Fluorescent Protein Biosensors
Genetically-encoded FRET biosensors are useful in detecting a many biological events.
Fluorescent Proteins
Fluorescent proteins are currently the probes of choice for live-cell imaging.
Fluorescent Speckle Microscopy
Stochastic but sparse labeling of macromolecules to track protein dynamics.
Fluorophore Co-Localization
Detecting the presence of two or more molecules at the same physical location.
Multiphoton Microscopy
Unique excitation scheme that reduces photobleaching and phototoxicity.
Single Molecule Fluorescence Microscopy
This technique is emerging as a powerful tool for superresolution imaging.
Spectral Imaging and Linear Unmixing
A powerful technique for co-localization, FRET, and removing autofluorescence.
Spinning Disk Microscopy
Excellent technique for high-speed imaging of living cells in real time with a CCD camera.
Synthetic Fluorophores and Hybrid Tags
A number of fluorophores have been developed for imaging living and fixed cells.
Total Internal Reflection Microscopy
TIRFM is useful for probing cellular processes that occur near the membrane.













