Confocal Microscopy
Confocal microscopy offers several advantages over conventional optical microscopy, including controllable depth of field, the elimination of image degrading out-of-focus information, and the ability to collect serial optical sections from thick specimens. The key to the confocal approach is the use of spatial filtering to eliminate out-of-focus light or flare in specimens that are thicker than the plane of focus. There has been a tremendous explosion in the popularity of confocal microscopy in recent years, due in part to the relative ease with which extremely high-quality images can be obtained from specimens prepared for conventional optical microscopy, and in its great number of applications in many areas of current research interest.
Review Articles
Introductory Confocal Concepts (日本語)
A discussion of point scanning and pinhole detection using photomultipliers.
Confocal Imaging Modes
Optical sectioning, live-cell imaging, and time-lapse sequence acquisition.
Aberrations in Confocal Microscopy
How the trade-offs involved in objective design can affect confocal microscopy.
Spectral Imaging and Linear Unmixing
Distinguishing individual spectral profiles of fluorophores within complex mixtures.
Confocal Reflection Microscopy
The investigation of opaque specimens can be coupled with standard techniques.
Specimen Preparation and Imaging
Fixation, permeabilization, and staining with immunofluorescence and synthetic dyes.
Critical Aspects of Confocal Microscopy
Laser alignment, photobleaching, probes, magnification, and other important concepts.
Three-Color Confocal Imaging
Confocal microscopy can be used to produce images of triple-labeled fluorescent samples.
Resonant Scanning in Laser Confocal Microscopy
Resonant scanning mirrors generate the speed for real-time imaging of living cells.
Interactive Tutorials
-
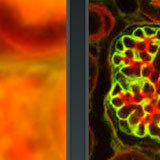
Laser Scanning Confocal Microscopy
A virtual microscope tutorial featuring a wide variety of specimens.
-
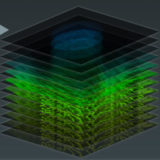
Lambda Stack Basic Concepts
The lambda stack is a three-dimensional dataset that consists of an image collection using the same specimen field acquired at different wavelength bands, each spanning a limited spectral region ranging from 2 to 20 nanometers.
-
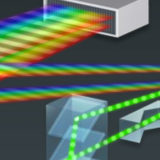
Nikon Diffraction Efficiency Enhancement System (DEES)
The purpose of the Nikon DEES system is to increase the efficiency of light diffraction by the gratings used to separate fluorescence emission into component wavelengths.
-

Integrated Circuit Inspection
Explore real-time confocal microscopy of integrated circuits by adjusting focus depth.
-
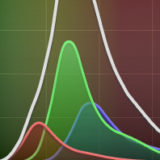
Spectral Imaging with Linear Unmixing
Discover how individual fluorophores can be identified within a complex mixture.
-
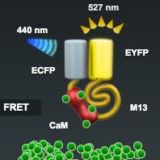
Spectral Imaging with FRET Biosensors
Spectral imaging is of significant advantage in separating the overlapping emission spectra of fluorescent proteins and other fluorophores in dynamic fluorescence resonance energy transfer (FRET) experiments, which are often complicated by the requirement for exceedingly fast image capture.
-
Lambda Stacks and Spectral Signatures
Learn how individual spectra are represented as narrow wavebands in lambda stacks.
Selected Literature References
Confocal Microscopy
Imaging with laser excitation and pinhole detection of fluorescence.
Fluorophore Co-Localization
Detecting the presence of two or more molecules at the same physical location.
Multiphoton Microscopy
Unique excitation scheme that reduces photobleaching and phototoxicity.
Resonant Scanning Confocal Microscopy
Resonant scanning confocals can gather images at 30 frames per second or higher.
Spectral Imaging and Linear Unmixing
A powerful technique for co-localization, FRET, and removing autofluorescence.
Spinning Disk Microscopy
Excellent technique for high-speed imaging of living cells in real time with a CCD camera.













