Dual Band Excitation: FITC-Texas Red
Ultraviolet and visible transmission spectral profiles for the Nikon FITC-Texas Red filter combination are illustrated below in Figure 1. This filter set is designed for optimal detection of signals from fluorescein isothiocyanate (FITC) and Texas Red probes in dual-labeling experiments, and incorporates an excitation filter with narrow bandpass windows in the blue (490-505 nanometers) and the green (560-580 nanometers) spectral regions. The dual emission (barrier) filter passbands incorporated into a single element allow detection of green and red emission from the two fluorochromes simultaneously. Sharp transitions between the transmission and reflection bands for each filter, and application of a dichromatic mirror having two bandpass transmission regions (polychromatic) chosen to be complementary to the emission and excitation wavelengths, permit dual-band signal detection with minimal interference.
Figure 1 - FITC-Texas Red Dual Band Excitation
Dual Excitation Filter Block FITC-Texas Red Specifications
- Excitation Filter Wavelengths: 490-505 nanometers (bandpass, 498 CWL) and 560-580 nanometers (bandpass, 570 CWL)
- Polychromatic Mirror Wavelengths: 510-555 nanometers (bandpass) and 585-665 nanometers (bandpass)
- Barrier Filter Wavelengths: 515-545 nanometers (bandpass, 530 CWL) and 600-650 nanometers (bandpass, 625 CWL)
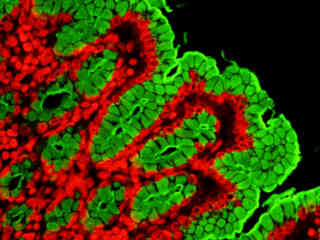
The FITC-Texas Red dual excitation band fluorescence filter combination is designed specifically for simultaneous detection of the fluorochromes FITC and Texas Red with minimal crosstalk between bands, and can be employed with other pairs of fluorescent probes having similar spectral profiles. The shorter wavelength signal channel of the filter set selects narrowly defined spectral regions for blue excitation and green emission detection, while the longer wavelength component corresponds to green excitation and red emission detection. The FITC-Texas Red filter set is recommended when studying various combinations of the following fluorophores: FITC, green fluorescent protein (GFP), Cy2, BODIPY FL, Oregon Green, or Alexa Fluor 488 (blue excitation), paired with Texas Red or Alexa Fluor 594 (green excitation). The images presented in Figure 2 demonstrate the performance of this filter combination with a variety of fluorescence probe pairs targeted at different intracellular locations.
Figure 2 - Nikon FITC-Texas Red Dual Excitation Filter Set
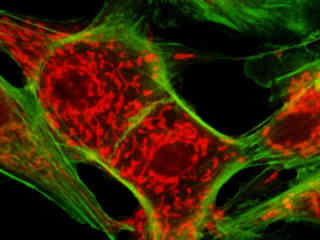
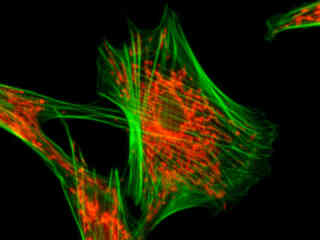
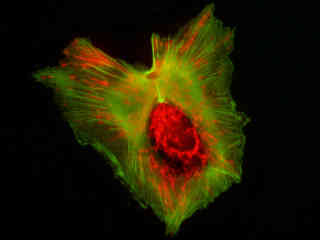
Illustrated in Figure 2(a) is the fluorescence emission from a culture of Madin-Darby canine kidney (MDCK line) cells stained with MitoTracker Red CMXRos and Alexa Fluor 488-phalloidin, which target the intracellular mitochondrial network and cytoskeletal actin filaments, respectively. The absorption maximum of MitoTracker Red CMXRos is 579 nanometers and the emission maximum occurs at 599 nanometers, while the corresponding values for Alexa Fluor 488 are 498 and 519 nanometers. In addition, the specimen was simultaneously stained with DAPI (targeting DNA in the cell nucleus; blue emission). Note the absence of signal from the blue (DAPI) fluorophore, but the bright orange-red fluorescence exhibited by the tubular mitochondria and the green emission from actin filaments in the cytoplasm.
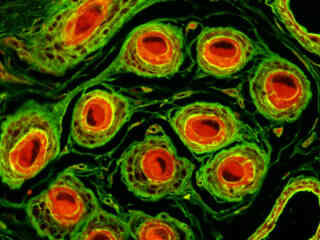
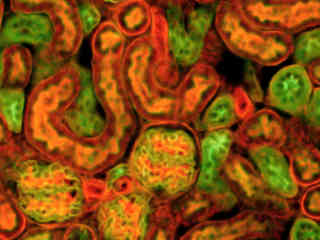
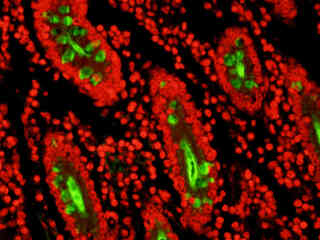
A thin section of sheep epidermal skin tissue stained for DNA and glycoproteins with SYTOX Orange and Oregon Green 488 wheat germ agglutinin, respectively, is presented in Figure 2(b). The absorption maximum of SYTOX Orange is 547 nanometers and the emission maximum occurs at 570 nanometers, while the corresponding values for Oregon Green 488 are 496 and 524 nanometers. In addition, the specimen was simultaneously stained with Alexa Fluor 350 conjugated to phalloidin (targeting the actin network; blue emission). Note the absence of signal from the blue (Alexa Fluor 350) fluorophore, but the bright orange-red fluorescence exhibited by the nuclei and the green emission from glycoproteins in the cytoplasm and membrane components of the hair follicles.
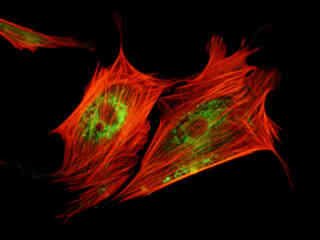
Fluorescence emission in a culture of Indian Muntjac deerskin fibroblast cells stained with Alexa Fluor 568 conjugated to phalloidin and LysoTracker Green DND-26, which target intracellular actin and lysosomes, respectively, are displayed in Figure 2(c). The absorption maximum of Alexa Fluor 568 is 578 nanometers and the emission maximum occurs at 603 nanometers, while the corresponding values for LysoTracker Green DND-26 are 501 and 511 nanometers. In addition, the specimen was simultaneously stained with DAPI (targeting DNA in the cell nucleus; excitation at 358 nanometers and emission at 461 nanometers). Note the absence of signal from the blue fluorophore (DAPI), but the presence of bright green and orange-red fluorescence exhibited by the lysosomes and actin filaments in the cytoskeletal network.
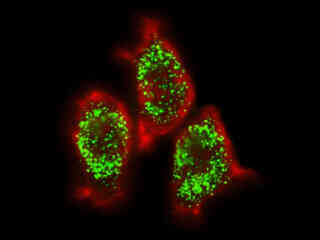
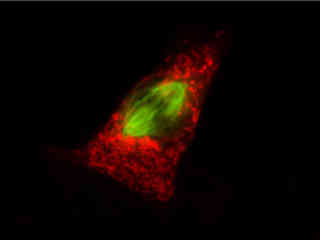
Figure 2(d) demonstrates the fluorescence emission from a culture of HeLa carcinoma cells transfected with an EGFP-peroxisomal targeting signal 1 (PTS1) fusion protein and stained with Alexa Fluor 546-phalloidin. These fluorescent probes target the peroxisomes and cytoskeletal actin filament network, respectively. The absorption maximum of the EGFP-PTS1 chimera is 488 nanometers and the emission maximum occurs at 507 nanometers, while the corresponding values for Alexa Fluor 546 are 556 and 573 nanometers. In addition, the specimen was simultaneously stained with Hoechst 33258 (targeting the DNA in the nucleus; blue emission). Note the absence of signal from the blue fluorophore, but the bright orange-red fluorescence exhibited by the cytoskeletal actin filaments and the intense green emission from peroxisomes in the cytoplasm.
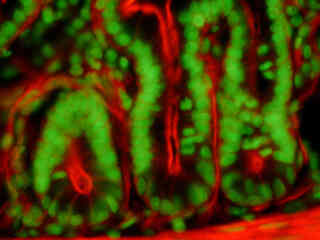
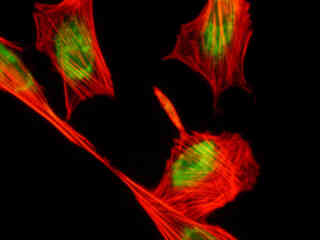
A thin section of rabbit lung tissue stained for actin filaments and glycoproteins with Alexa Fluor 546-phalloidin and Oregon Green 488 wheat germ agglutinin, respectively, is illustrated in Figure 2(e). The absorption maximum of Alexa Fluor 546 is 556 nanometers and the emission maximum occurs at 573 nanometers, while the corresponding values for Oregon Green 488 are 496 and 524 nanometers. In addition, the specimen was simultaneously stained with DAPI (targeting DNA in the nucleus; blue emission). Note the absence of signal from the blue fluorophore, but the bright orange-red fluorescence exhibited by filamentous actin and the green emission from glycoproteins in the cytoplasm and membrane components of the tissue section.
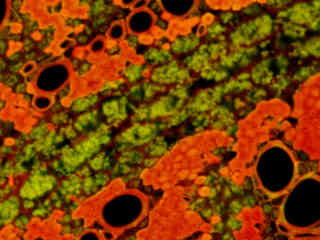
Autofluorescence emission intensity from a thin section of alfalfa (Medicago sativa) root tissue is demonstrated in Figure 2(f). Endogenous autofluorescence in plant tissues arises from a variety of biomolecules, including lignins, chlorophyll, carotene, and xanthophyll. In the blue and green excitation regions, chlorophyll has an absorption band with a high extinction coefficient and produces a significant amount of fluorescence when excited with wavelengths between 450 and 550 nanometers. For the alfalfa root tissue illustrated above, note the presence of autofluorescence emission intensity in the green and red spectral regions, which is characteristic of the Nikon FITC-Texas Red fluorescence filter combination.