Violet Excitation Filter Sets
Included in the Nikon violet excitation fluorescence filter portfolio are three combinations that contain either bandpass or longpass emission (barrier) filters capable of selectively isolating fluorescence emission through either a narrow or wide region of the blue, green, and red wavelengths. These combinations cover an excitation wavelength range between 379-420 nanometers with bandpass width profiles of 10, 22, and 40 nanometers. Two of the combinations employ the same dichromatic mirror, while the third set has a mirror with a lower wavelength cut-on to coincide with its shorter-wavelength excitation band.
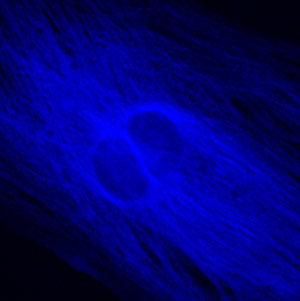
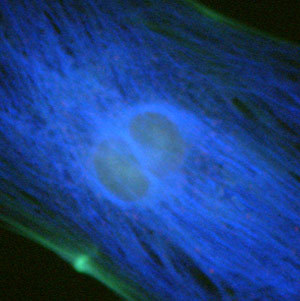
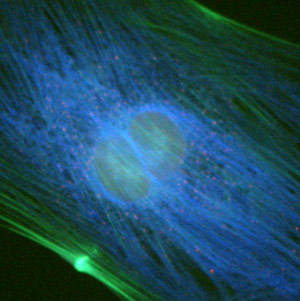
Figure 1 - Violet Excitation Filter Sets
Performance of the violet filter sets can be judged by comparing images from the same viewfield captured with each of the individual filter combinations, as presented in Figure 1. The specimen is a culture of Indian Muntjac fibroblast deerskin cells that were immunofluorescently labeled with primary anti-bovine alpha-tubulin mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to Pacific Blue. The visible light absorption maximum of Pacific Blue is 410 nanometers and the emission maximum occurs at 455 nanometers. Note the prominent staining of the intracellular microtubular network that extends throughout the cytoplasm. In addition, the specimen was simultaneously stained for F-actin with Alexa Fluor 488 conjugated to phalloidin, and for mitochondria with MitoTracker Red CMXRos. Note the absence of signal from the red fluorophore (MitoTracker Red) in Figures 1(b) and 1(c), but the presence of green fluorescence from several of the more prominent actin cytoskeletal elements. The medium band excitation filter combination (V-2A; Figure 1(c)) exhibits a much higher background signal than the narrow bandpass filter (V-1A; Figure 1(b)), which, in turn, produces more background fluorescence than the bandpass blue fluorescent (BFP) protein set (Figure 1(a)).
Commonly specified as the standard violet filter set, the V-2A combination is equipped with a 40-nanometer bandpass excitation filter that covers the majority of the violet wavelength region. Coupled with a 430-nanometer cut-on dichromatic mirror and a longpass emission filter, the V-2A produces the brightest images of any filter combination in the Nikon violet group (Figure 1(c)). Specifications for the dichromatic mirrors and filters from the various Nikon violet filter combinations are listed in Table 1.
Table 1 - Nikon Violet Filter Combination Specifications
| Filter Set Description | Excitation Filter (nm) | Dichromatic Mirror (nm) | Barrier Filter (nm) | Remarks |
|---|---|---|---|---|
| V-1A | 405/10 (400-410) | 430 (LP) | 435 (LP) | Narrow Excitation Band Longpass Emission Filter |
| V-2A | 400/40 (380-420) | 430 (LP) | 450 (LP) | Medium Excitation Band Longpass Emission Filter |
| BFP | 390/22 (379-401) | 420 (LP) | 460/50 (435-485) | Narrow Excitation Band Bandpass Emission Filter |
- V-1A - The V-1A filter combination is designed as a filter block for violet fluorescence excitation with a narrow bandpass region to minimize autofluorescence. The longpass emission filter allows detection of a wide range of fluorochrome wavelengths.
- V-2A - The V-2A combination is designed as a standard violet fluorescence filter block having the greatest degree of versatility. With a medium excitation bandpass and longpass emission filter, the images produced are the brightest of the Nikon violet filter group.
- BFP (Blue Fluorescent Protein) - The BFP filter block combines a narrow excitation band with a bandpass emission filter to limit autofluorescence as well as to exclude detection of emission from red and green fluorophores. This filter combination is excellent for imaging blue fluorescent protein derivatives.
A wide array of fluorophores has been developed for investigations using excitation wavelengths spanning the violet region. Catalogued in Table 2 are some of the most popular dyes and fluorescent probes that can be visualized with the Nikon violet filter combinations. The localized environment significantly influences fluorophore absorption and emission spectra maximum (peak) wavelengths, so the values presented in Table 2 may vary with experimental conditions. This list is intended to serve only as a guide for filter and fluorophore selection and should not be considered a comprehensive or exhaustive compilation. Many of the fluorescent probes included in Table 2 are proprietary and have been developed to minimize photobleaching while ensuring a maximum overlap between the fluorochrome absorption and emission spectra and common fluorescence filter combinations. Note that due to broad absorption and emission bands, several of the fluorescent probes listed in Table 2 are also suitable for use with filter combinations in other excitation wavelength regions, including ultraviolet and blue-violet.
Table 2 - Fluorochromes with Violet Excitation Spectral Profiles
| Fluorochrome | Excitation Wavelength (Nanometers) | Emission Wavelength (Nanometers) | Recommended Filter Set(s) |
|---|---|---|---|
| ACMA (Aminochloromethoxyacridine) | 430 | 474 | V-2A |
| Acriflavin | 436 | 520 | V-2A |
| AFA (Acriflavin Feulgen SITSA) | 355-425 | 460 | V-2A, BFP |
| Alexa Fluor 405 | 401 | 421 | V-2A, V-1A |
| Alexa Fluor 430 | 433 | 541 | V-2A |
| Berberine Sulfate | 430 | 550 | V-2A |
| Beta-Lactamase | 409 | 447-520 | V-2A, V-1A |
| BFP (Blue Fluorescent Protein) | 381 | 445 | BFP |
| BFP/GFP FRET | 382 | 508 | V-2A |
| Bimane | 398 | 490 | V-2A |
| Bis-ANS (Bis-Anilinonaphthalene Sulfonic Acid) | 395 | 500 | V-2A |
| Bis-BTC (Bis-Benzothiazole Indicator) | 405 (High Ion) 455 (Low Ion) | 505 (High Ion) 529 (Low Ion) | V-2A, V-1A |
| Blancophor FFG | 390 | 470 | V-2A, BFP |
| Brilliant Sulphoflavin FF | 430 | 520 | V-2A |
| BTC (Benzothiazole Indicator) | 401 (High Ion) 464 (Low Ion) | 529 (High Ion) 533 (Low Ion) | V-2A, V-1A |
| BTC-5N (Benzothiazole Indicator) | 417 (High Ion) 459 (Low Ion) | 532 (High Ion) 517 (Low Ion) | V-2A |
| Calcofluor White | 365-380 | 435-475 | BFP, V-1A |
| Cascade Blue | 377, 399 | 423 | BFP, V-1A |
| Cascade Yellow | 400 | 550 | V-2A, V-1A |
| Catecholamine | 410 | 470 | V-2A, V-1A |
| CCF2 (GeneBLAzer) | 402 | 520 | V-2A, V-1A |
| Coumarin | 384 | 470 | V-2A, BFP |
| CPM (Coumarin-Maleimide) | 385 | 471 | V-2A, BFP |
| CTC (Chlorotetracycline) | 400-450 | 600-630 | V-2A, V-1A |
| DAMC (Diethylaminomethylcoumarin) | 388 | 520 | V-2A, V-1A |
| DAPI (Diamidinophenylindole) | 358 | 461 | All |
| Dapoxyl | 373 | 574 | V-2A, V-1A |
| Diphenyl Brilliant Flavine 7GFF | 430 | 520 | V-2A |
| EBFP (Enhanced Blue FP) | 383 | 447 | V-2A, BFP |
| ECFP (Enhanced Cyan FP) | 433 | 475 | V-2A |
| ERTracker Blue/White DPX | 374 | 575 | V-2A, V-1A |
| Euchrysin | 430 | 540 | V-2A |
| Flazo Orange | 375-530 | 612 | V-2A |
| Fluorescamine | 390 | 475 | V-2A, BFP |
| FluoroJade | 425 | 510 | V-2A |
| FIF (Formaldehyde Induced Fluorescence) | 405 | 435 | V-1A, V-2A |
| GFP (Wild Type) | 395 | 508 | V-2A |
| GFP (Blue Shifted Y66H) | 382 | 448 | All |
| Genacryl Brilliant Yellow 10GF | 430 | 485 | V-2A |
| Genacryl Yellow 5GF | 430 | 475 | V-2A |
| Gloxalic Acid | 405 | 460 | V-2A, V-1A |
| HAT (Hydroxytryptamine) | 370-415 | 520-540 | V-2A, V-1A |
| Hoechst 34580 | 392 | 440 | All |
| HPTS (Hydroxypyrene Trisulfonic Acid) | 403 | 513 | V-2A, V-1A |
| Hydroxycoumarin | 386 | 448 | V-2A, BFP |
| Leucophor SF | 380 | 465 | V-2A, BFP |
| Leucophor WS | 395 | 465 | All |
| Lucifer Yellow CH | 425 | 528 | V-2A |
| Lucifer Yellow VS | 430 | 535 | V-2A |
| LysoSensor Blue DND-192, DND-167 | 374 | 425 | V-2A, V-1A |
| LysoSensor Green DND-153, DND-189 | 442 | 505 | V-2A |
| LysoSensor Yellow/Blue | 329 (High pH) 384 (Low pH) | 440 (High pH) 540 (Low pH) | V-2A, V-1A |
| LysoTracker Blue | 373 | 422 | All |
| Marina Blue | 365 | 460 | All |
| Methylumbelliferone | 385 | 502 | V-2A |
| Mithramycin | 395-450 | 535-570 | V-2A, V-1A |
| Monobromobimane | 380 | 461 | V-2A, V-1A |
| Nuclear Fast Red | 289-530 | 580 | V-2A, V-1A |
| Olivomycin | 430 | 545 | V-2A |
| Pacific Blue | 410 | 455 | All |
| PBFI (Benzofuran Isophthalate) | 340/380 | 557/507 | V-2A |
| Phorwites | 360-380 | 430 | All |
| POPO-1, PO-PRO-1 | 434 | 456 | V-2A |
| Primuline O | 410 | 550 | V-2A, V-1A |
| PyMPO (pyridyloxazole) | 415 | 570 | V-2A, V-1A |
| Pyronine | 410 | 540 | V-2A, V-1A |
| QDots 525-655 | 350-450 | 525-655 | V-2A, V-1A |
| Quinacrine Mustard | 423 | 503 | V-2A |
| Sapphire GFP | 395 | 511 | V-2A, V-1A |
| Serotonin | 365 | 520-540 | V-2A, V-1A |
| Sevron Orange | 440 | 530 | V-2A |
| Sevron Yellow L | 430 | 490 | V-2A |
| SGBFP (Super Glow BFP) | 387 | 450 | BFP, V-2A |
| Spectrum Aqua | 433 | 480 | V-2A |
| Spectrum Blue | 400 | 450 | All |
| SYTO 40 | 420 | 441 | V-2A, V-1A |
| SYTOX Blue | 444 | 480 | V-2A, V-1A |
| Tetracycline | 390-425 | 525-560 | V-2A, V-1A |
| Thioflavin S | 430 | 550 | V-2A |
| Thiolyte | 370-385 | 477-484 | V-2A, BFP |
| Tinopol CBS | 390 | 430 | All |
| TNS (Toluidinylnaphthalenesulfonate) | 380 | 483 | V-2A, BFP |
| Uranine B | 420 | 520 | V-2A, V-1A |
Although the three filter combinations described above adequately serve in a majority of the investigations with violet wavelengths, several additional special filter sets are available from the aftermarket manufacturers. Some of these combinations incorporate violet-band excitation with non-standard dichromatic mirrors and barrier filters, which may be chosen, in some instances, to match particular detector characteristics. Filter sets designed for xenon arc-discharge lamp excitation generally employ wide bandpass excitation filters (approximately 100 nanometers) centered in the 380-420 nanometer (violet) spectral region. In other variations, a narrow excitation bandpass may be designed to selectively isolate specific emission lines of sources such as mercury lamps or lasers, which occur in the appropriate spectral region.
Other specialized filter sets intended for ratiometric analysis of various environment-sensitive probes include two emission filters with distinct bandpass regions. Sets for ion-sensitive probes, such as beta-lactamase, can be configured in two variations, each having a single excitation filter and dual emission filters, while utilizing different dichromatic mirror complements. As configured for simultaneous imaging of both emission wavelengths with an emission-splitting system, two dichromatic mirrors are included in the filter set. Only one dichromatic mirror is utilized for sequential ratiometric imaging with an emission filter wheel. In addition, filter combinations for specific fluorophores have been developed, some of which are designed to allow rapid dual imaging with a single emission filter and two excitation filters. Examples of these are special sets for dual excitation of sapphire and yellow fluorescent proteins, pH-sensitive green fluorescent protein, and cyan and yellow fluorescent proteins, each of which has excitation wavelengths in the violet spectral region.