Blue Excitation: B-1E (Bandpass Emission)
Ultraviolet, visible, and near-infrared transmission spectral profiles for the excitation, barrier, and dichromatic mirror interference filters in the Nikon B-1E combination are illustrated below in Figure 1. This filter set is designed to combine a narrow passband excitation range (20 nanometers) with a bandpass emission filter in order to provide high signal-to-noise ratio, enhanced by exclusion of undesired emission wavelengths. The emission filter has a center wavelength of 540 nanometers (40-nanometer bandpass), and in similarity to the other filter combinations in this group, the B-1E set employs a longpass dichromatic mirror with a cut-on wavelength of 505 nanometers.
Figure 1 - B-1E (Narrow Band Blue Excitation)
Blue Excitation Filter Block B-1E Specifications
- Excitation Filter Wavelengths: 470-490 nanometers (bandpass, 480 CWL)
- Dichromatic Mirror Cut-on Wavelength: 505 nanometers (longpass, LP)
- Barrier Filter Wavelengths: 520-560 nanometers (bandpass, 540 CWL)
The B-1E filter combination is specifically designed with an excitation bandpass suitable for imaging specimens labeled with fluorescein isothiocyanate (FITC) or closely related derivatives, and also incorporates a bandpass barrier filter in order to exclude yellow, orange, and red fluorescence signals. This emission filter is identical to that included in the B-2E set, but the bandpass center wavelength is longer than that of the B-2E/C combination. Among the Nikon blue excitation filter sets, those in the E series produce images with a much higher signal-to-noise ratio than do combinations with longpass emission filters (the A series). In addition, the image background regions appear significantly darker with the bandpass filter combinations. Fluorescence emission occurring at longer wavelengths than green (the yellow, orange, and red spectral regions) is excluded from images acquired using the B-1Efilter combination (See Figure 2).
The B-1E set is recommended when investigating the following fluorophores: Acridine Orange bound to DNA (excludes detection of Acridine Orange bound to RNA), Acridine Yellow, Alexa Fluor 488, BODIPY probes, Calcein, Calcium Green, carboxyfluorescein (FAM), coriphosphine O, Cy2, enhanced green and yellow fluorescent proteins (EGFP and EYFP), red-shifted green fluorescent protein (rsGFP), Fluo-3, fluorescein isothiocyanate (FITC), several LysoTracker derivatives, MitoTracker Green, Oregon Green derivatives, oxacarbocyanine (DiO) probes, Rhodamine 123 (and other derivatives), Spectrum Green, SYTO probes, SYTOX Green, YO-PRO 1, and YOYO 1. The images presented in Figure 2 demonstrate the performance of this filter combination with a variety of blue light absorbing fluorescence probes targeted at different intracellular locations.
Figure 2 - Nikon B-1E Narrow Band Blue Excitation Bandpass Filter Set
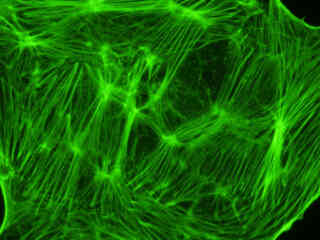
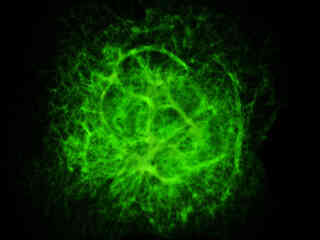
Presented in Figure 2(a) is the fluorescence emission intensity from a culture of bovine pulmonary artery endothelial cells stained with BODIPY FL phallacidin, which binds to the intracellular filamentous actin network. The absorption maximum of BODIPY FL is 503 nanometers and the emission maximum occurs at 512 nanometers. In addition, the specimen was simultaneously stained with DAPI (targeting DNA in the cell nucleus; blue emission) and MitoTracker Red CMXRos (targeting mitochondria; red emission). Note the absence of signal from the red (MitoTracker) and blue (DAPI) fluorophores, but the bright green fluorescence exhibited by the actin filaments.
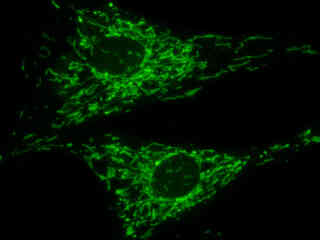
Figures 2(b) and 2(c) illustrate Indian Muntjac deerskin fibroblast cells labeled with different staining regimens. The cells in Figure 2(b) were immunofluorescently labeled with primary anti-oxphos complex V inhibitor protein monoclonal antibodies (mouse) followed by goat anti-mouse Fab fragments conjugated to Alexa Fluor 488. The absorption maximum of Alexa Fluor 488 is 495 nanometers and the emission maximum occurs at 519 nanometers. In addition, the specimen was simultaneously stained for F-actin with Alexa Fluor 350 (blue) conjugated to phalloidin, and for DNA with SYTOX Orange. Note the absence of signal from both the blue and orange fluorophores, but the significant emission intensity from Alexa Fluor 488, which has efficiently labeled intracellular mitochondria. In many cases, SYTOX Orange stains a variety of cytoplasmic elements in addition to DNA, but this is not evident in the image.
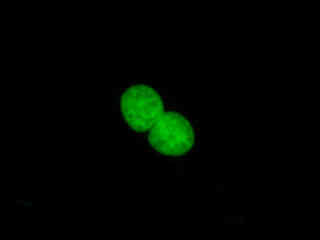
Indian Muntjac cells displayed in Figure 2(c) were labeled with SYTOX Green to stain chromatin in the nuclei. The absorption maximum of SYTOX Green is 504 nanometers and the emission maximum occurs at 523 nanometers. In addition, the specimen was simultaneously labeled with primary anti-human OxPhos Complex V inhibitor protein mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to Pacific Blue (absorption maximum at 410 nanometers). The actin cytoskeletal network was also labeled with phalloidin conjugated to Alexa Fluor 568. Note the absence of signals from the red (Alexa Fluor 568) and blue (Pacific Blue) fluorophores, but the presence of intense green fluorescence from the interphase cellular nuclei.
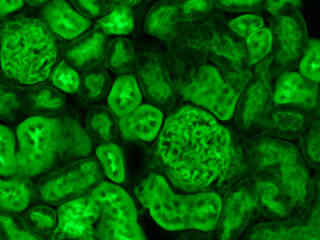
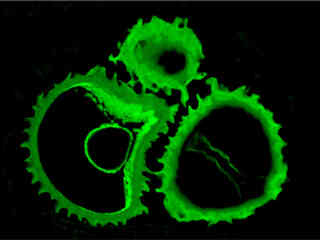
Fluorescence emission intensity from a thin section of mouse kidney stained with multiple fluorophores is displayed in Figure 2(d). Nuclei in the tissue section were targeted with the nucleic acid probe DAPI, which has an excitation maximum at 358 nanometers and an emission maximum at 461 nanometers when bound to DNA in cell cultures and tissue sections. In addition, the cryostat section was also simultaneously stained with Alexa Fluor 488 wheat germ agglutinin (glomeruli and convoluted tubules) and Alexa Fluor 568 phalloidin (filamentous actin and the brush border). Note the absence of signal from both the red (Alexa Fluor 568) and blue (DAPI) fluorophores.
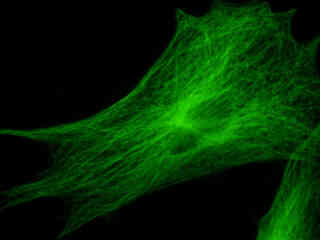
A culture of rat kangaroo (PtK2) epithelial cells that were immunofluorescently labeled with primary anti-bovine alpha-tubulin mouse monoclonal antibodies followed by goat anti-mouse Fab fragments conjugated to Alexa Fluor 488 is presented in Figure 2(e). Staining of the intracellular microtubule network that extends throughout the cytoplasm is very evident in the image. In addition, the specimen was simultaneously stained for the nuclear protein cdc6 (conjugated to Pacific Blue), and for F-actin with phalloidin conjugated to Alexa Fluor 568. Note the absence of signals from the blue (Pacific Blue) and red (Alexa Fluor 568) fluorophores, but the presence of intense green fluorescence from several of the more prominent tubulin cytoskeletal elements.
Figure 2(f) demonstrates autofluorescence emission intensity from a thin section of fern strobilus (Cyrtomium falcatum) tissue. Endogenous autofluorescence in plant tissues arises from a variety of biomolecules, including chlorophyll, carotene, and xanthophyll. In the blue region, chlorophyll has an absorption band with a high extinction coefficient and produces a significant amount of fluorescence when excited with wavelengths between 420 and 460 nanometers. Note the absence of spectral bleed-through from autofluorescence emission in the blue and red spectral regions with the B-1E filter combination